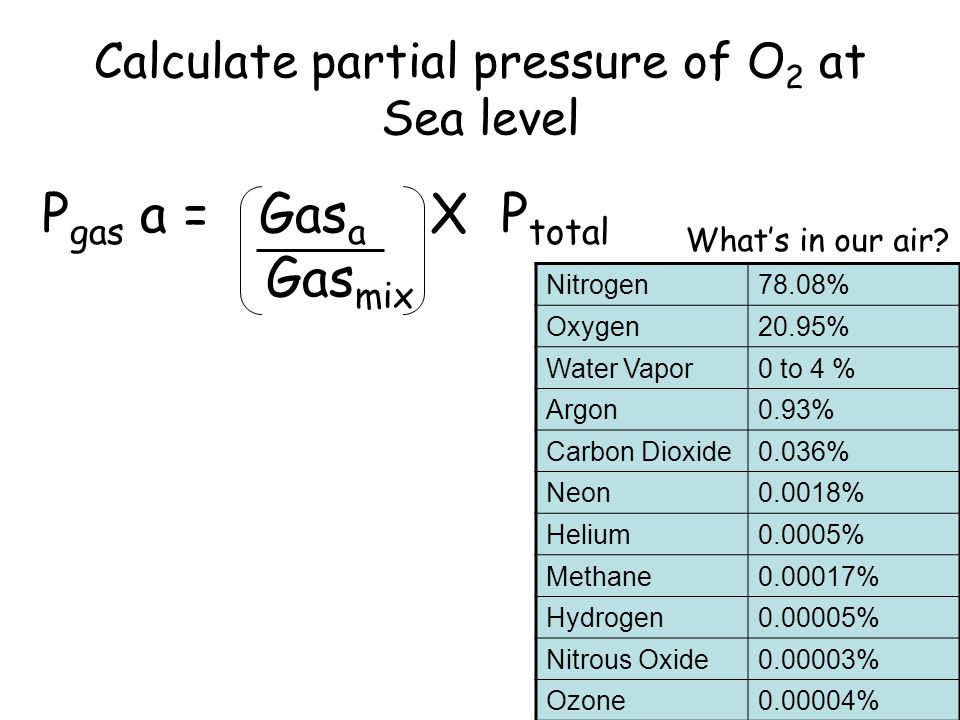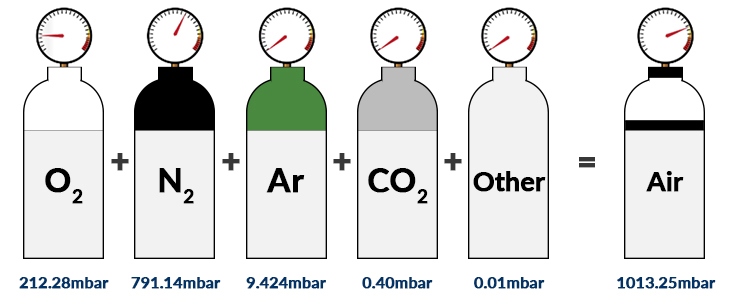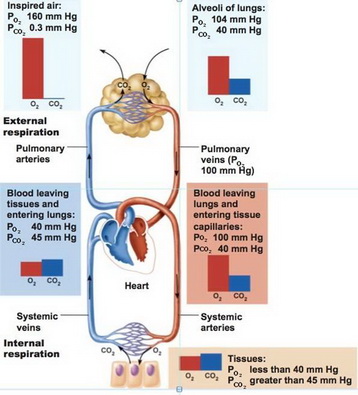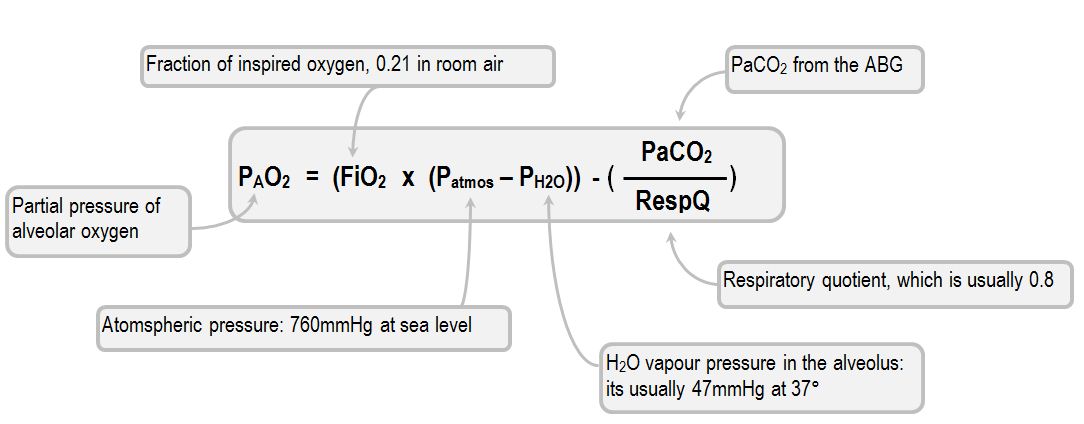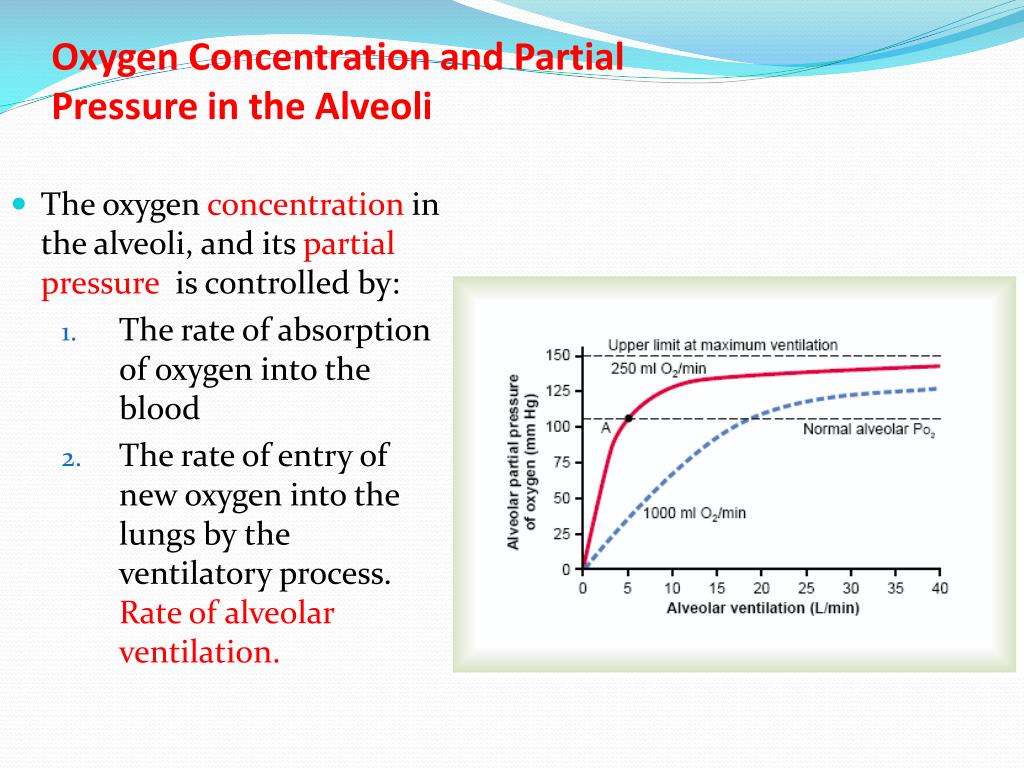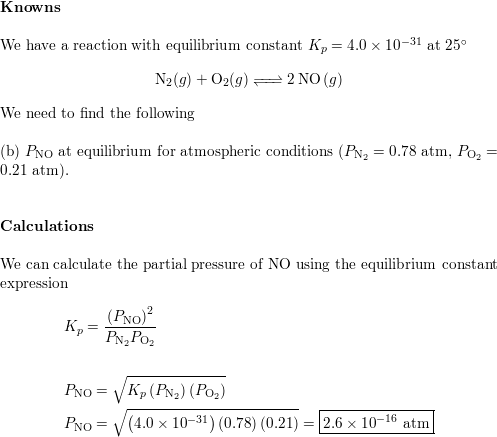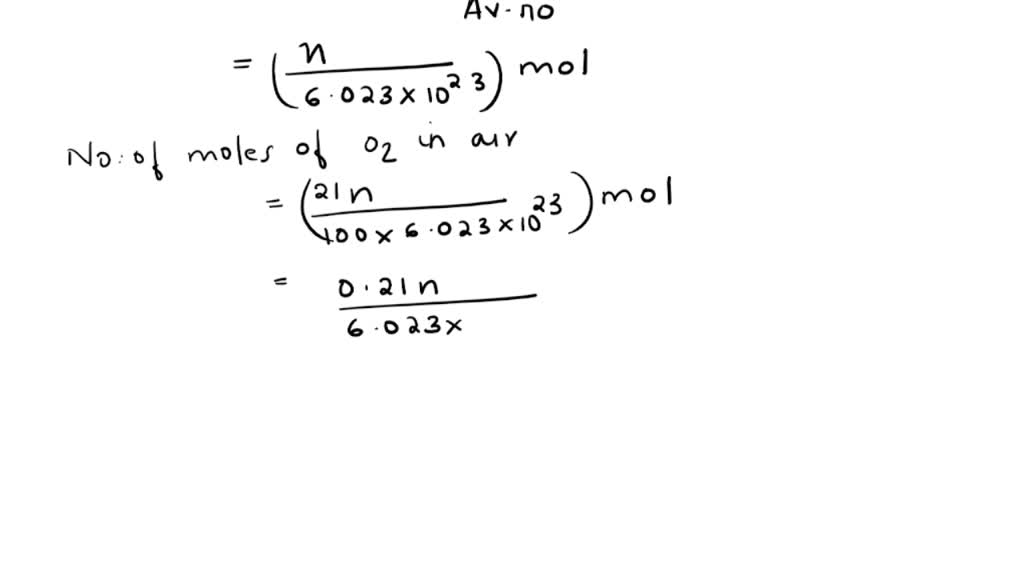
SOLVED: calculate the partial pressure of O2 (in atm) in dry air when the atmospheric pressure is 754 mmHg.
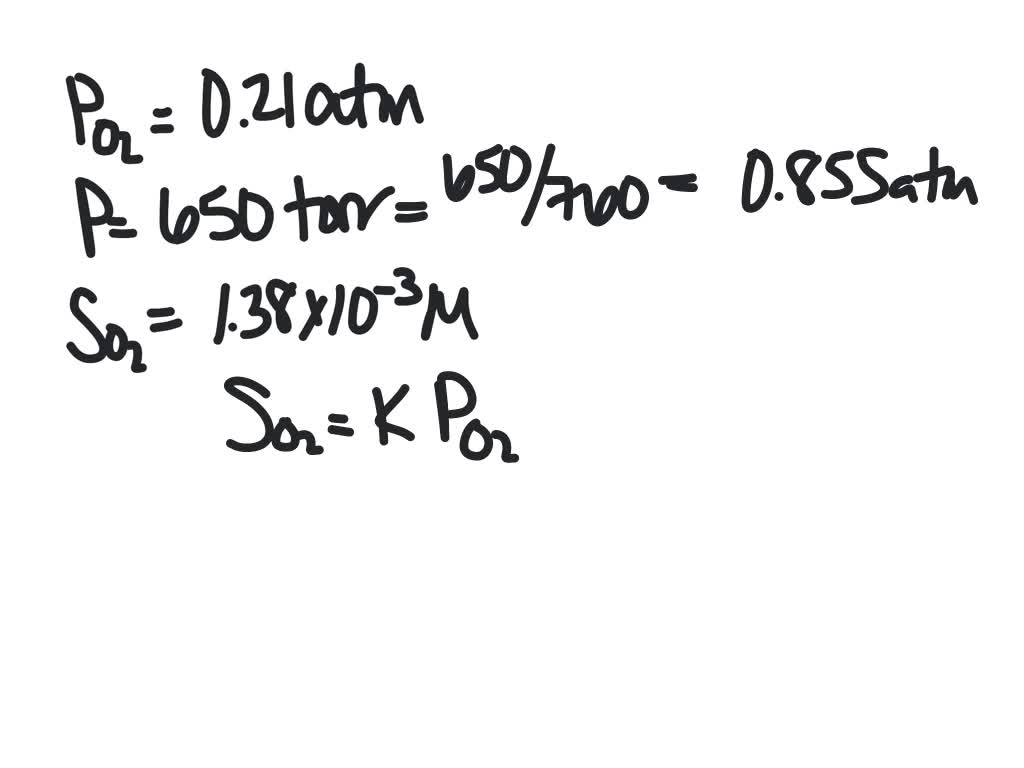
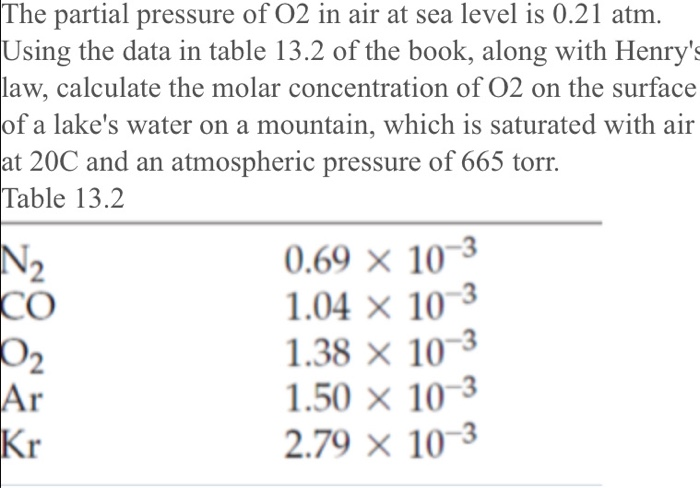
SOLVED:The partial pressure of O2 in air at sea level is 0.21 atm . Using the data in Table 13.1 , together with Henry's law, calculate the molar concentration of O2 in

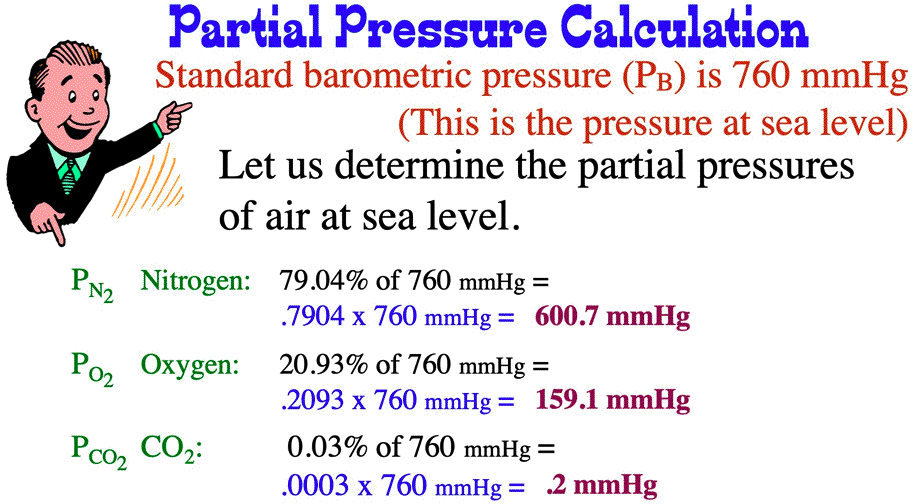
SOLVED: Calculate the partial pressure of each of the following gases in room. Air when the barometric pressure is 760 mmHg ( assume the room air contains 21% oxygen, 78% nitrogen, and
![AP chemistry: partial pressures]How do I calculate the partial pressure and the volume of the jar? : r/HomeworkHelp AP chemistry: partial pressures]How do I calculate the partial pressure and the volume of the jar? : r/HomeworkHelp](https://i.redd.it/4xvi44skj3y51.jpg)
AP chemistry: partial pressures]How do I calculate the partial pressure and the volume of the jar? : r/HomeworkHelp